purpose
The feasibility of simultaneous determination of vitamins A and E in infant formula with UtraPerformance Convergence Chromatography ™ (UPC² ™).
background
The traditional steps for the determination of fat-soluble vitamins in food include: first, tedious sample preparation (such as saponification and extraction), and then high-performance liquid chromatography (HPLC) to detect by ultraviolet or fluorescence detector. 1 Since the sample preparation requirements for vitamins A and E are similar, the analysis of the two is usually performed simultaneously. 1 Recently, it was planned to apply a simplified sample preparation method without saponification reaction to the analytical method for the simultaneous determination of vitamin A and E in infant formula (IF). This method requires only one injection to transfer different forms of vitamin A Separate and quantify with E. 2 Omitting the saponification reaction process can greatly improve the analytical throughput. However, the chromatographic analysis time is still very long (25 min), and there is no written evaluation of the resolution of cis and trans vitamin A. Waters® Ultra High Performance Convergence Chromatography (UPC²) has become the third type besides LC and GC due to the unique properties of supercritical carbon dioxide (such as low viscosity, high diffusivity, and liquid-like solubility) The separation method can deal with a wider range of analysis problems. 3,4 In order to study the performance of UPC² for simultaneous determination of vitamins A and E, we conducted a feasibility analysis experiment on IF samples purchased from the market.
UPC² technology provides a quick and simple “green†method for simultaneous determination of vitamins A and E in infant formula.
solution
In the feasibility study experiment of this paper, firstly, the liquid-liquid extraction method was used to extract vitamin A (retinyl acetate and retinyl palmitate) and vitamin E (α-tocopheryl acetate) in the IF sample. And α-tocopherol); Then, the ACQUITY UPC² system equipped with a PDA detector was used for analysis. The column used was ACQUITY UPC² HSS C18 SB 3.0 × 100 mm, 1.8 μm, and gradient elution was performed using a mixture of carbon dioxide and methanol as the mobile phase (methanol ratio increased from 3% to 10%). The chromatograms of these compounds were extracted from the PDA data, and the maximum absorption wavelengths of retinol ester, α-tocopheryl acetate and α-tocopherol were 320nm, 283nm and 293nm in sequence, as shown in Figure 1.
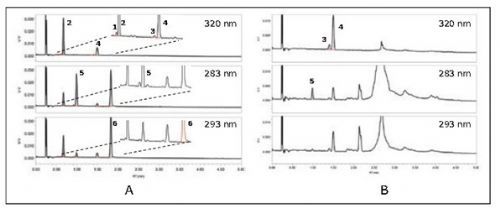
Figure 1. Typical chromatograms of vitamins A and E analyzed with UPC² / PDA. (A) Standard product; (B) Infant formula sample. Chromatographic peaks: 1 cis-retinyl acetate, 2 all-trans-retinyl acetate, 3 cis-retinyl palmitate, 4 all-trans-retinyl palmitate, 5 Alpha-tocopheryl acetate and 6 alpha-tocopherol.
With UPC², vitamins A and E can be separated quickly and perfectly. All compounds (including cis and trans retinyl esters) are separated from each other, and all elute before the peak of the sample matrix. The total run time for each injection is 8 minutes, which includes column equilibration time. Compared with the run time required by other methods (typically 25 minutes), the analysis speed is at least 3 times higher. The linearity, sensitivity, repeatability and recovery results of the analytical method are shown in Table 1 and Table 2. Although the sample extract can be directly injected, the LOQ results indicate that certain compounds need to be evaporated and concentrated, depending on the level of the compound in the IF sample. In this study, when measuring vitamin E, the sample extract was concentrated by 10 times by evaporation. UPC² is an environmentally friendly "green" technology. The main mobile phase carbon dioxide used in the analysis comes from the recovered carbon dioxide released by other industries, so the use of carbon dioxide in the experiment will not generate new greenhouse gases. When using UPC2 analysis, the consumption of modifier (methanol) per injection is only 0.9 mL, which is at least 90% lower than the consumption of 10 mL of hexane described in Reference 2.
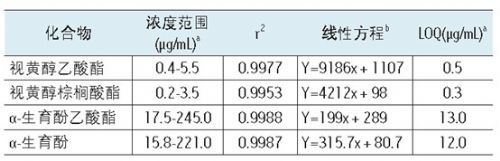
Table 1. Linearity and estimated LOQ of the method obtained by analysis using the UPC² / PDA system. a This concentration is the number of μg of analyte per mL of solution. b Y, peak area; x, concentration (μg / mL).

Table 2. Repeatability and recovery results obtained from spiked infant formula samples. a This value is the number of μg of vitamins per gram of infant formula.
to sum up
This experiment uses Waters ACQUITY UPC² / PDA system, equipped with a UPC² column, and can analyze cis and trans retinyl palmitate, cis and trans in commercial IF samples with only one injection Formula retinol acetate, α-tocopheryl acetate and α-tocopherol.
The sample analysis time is 8 min, which is three times faster than the traditional analysis time; the solvent consumption per injection is 0.9 mL, which is 1/10 of the solvent consumption of the normal phase LC method. The results obtained by the analysis method in this paper have good resolution, linearity, sensitivity, precision and accuracy. Therefore, UPC² technology has great potential advantages in becoming a practical solution for routine determination of vitamins A and E in infant formula products.
references
1. DeVries JW, Silvera K R. Determination of vitamins A (retinol) and E (alpha-tocopherol) in foods by
liquid c hromatography: collaborative study. J. AOAC International. 2002; 85: 424.
2. C havez-Servin JL, Castellote AI, Lopez-Sabater MC. Simultaneous analysis of Vitamins A and E in
infant milk-based formulae by normal-phase high-performance liquid chromatography-diode array
detection using a short narrow-bore column. J. Chromatogr. A. 2006; 1122: 138.
3. Aubin A. Analysis of Fat-Solu ble Vitamin C a psules Using Ultra Performanc e C onvergenc e Chromatogra p hy UP C.2 Waters Applic ation Note 720004394en.2012 June.
4. ACQUITY UPC² System broc hure.2012. P / n 720004225en.2012 Marc h.
Frequent use of the mouse and keyboard, arm position too high or too low can cause injury to the wrist. Keyboard Tray can solve this problem for you, you can freely adjust the position of the mouse keyboard. When operating a computer, the hand is slightly lower than the desktop, the upper and lower arms remain consistent, conducive to correct posture, reduce chronic strain, more ergonomic.
Our keyboard tray has the following advantages: 1.More room for manipulation 2. Easy storage and space saving; 3. Adjustable keyboard tilt Angle; 4. The upper and lower heights can be adjusted; 5, excellent texture, waterproof and easy to scrub;

Keyboard Tray
Under-Desk Keyboard Tray, Computer Keyboard Tray, Adjustable Keyboard Tray
Ningbo YINGBOTE Trading Co.,Ltd , http://www.intelligentoffice-cn.com